TenJet Patient Registry
Please join us in the research on TenJet
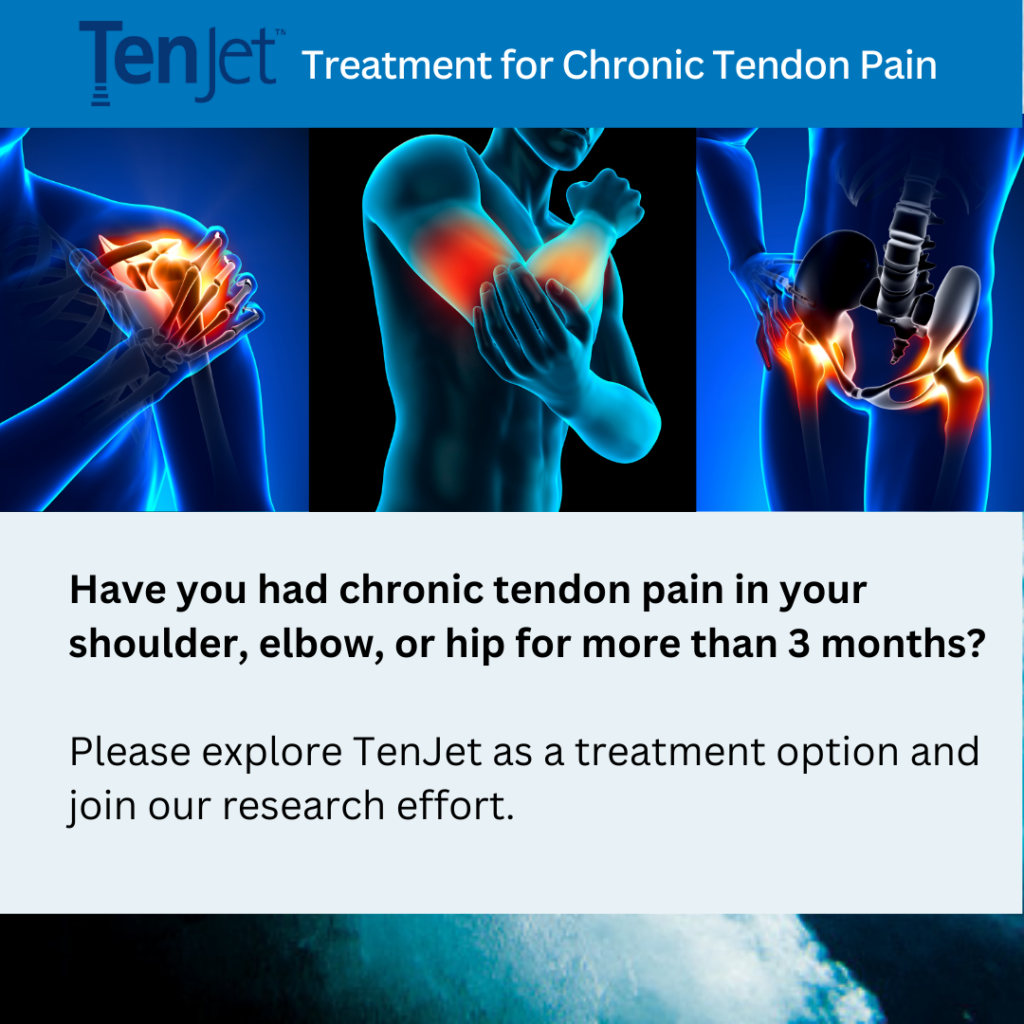
The TenJet Patient Registry will help us systematically gather and analyze data to understand how effective TenJet is in treating chronic tendinopathy in the elbow, hip, and shoulder.
Frequently Asked Questions
What is the TenJet Patient Registry?
The TenJet Patient Registry seeks to collect 2-year outcomes data in 600 patients treated with the TenJet device across 3 academic institutions in the United States.
What is the goal of the TenJet Patient Registry?
The goal of the TenJet Patient Registry is to systematically gather clinical evidence over a period of 2 years on changes experienced by the patient in their pain and activity level after treatment. This research will add to the knowledge and understanding of the effectiveness of the procedure in treating patients with chronic tendinopathy.
What does it mean for me to join in the research project?
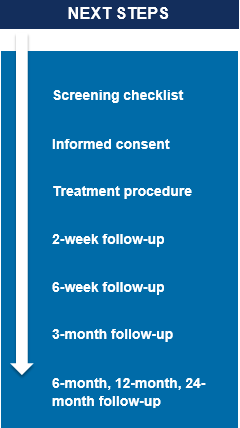
Before treatment and 6 times after treatment, at 2 weeks, 6 weeks, 3 months, 6 months, 1 year, 2 years; you will be asked to fill questionnaires with questions related to your pain, activities of daily living and/or recreation, and quality of life.
Your physician will also recommend you follow either a post- procedure home exercise protocol or go for physical therapy after the procedure.
How long will it take me to fill out the questionnaires?
It should not take you more than 15 minutes to fill out the questionnaires.
Do I have to come to the doctor’s office to fill out the questionnaire?
You do not have to come to the doctor’s office to fill out the questionnaires unless you have an in-person doctor’s appointment. The questions could be answered during a phone interview, digitally or by mail.
However, as is standard practice, you will be expected to return to your doctor’s office for a follow-up visit within 2 weeks of the procedure to check the incision and determine if you are ready to start a rehabilitation or home exercise program.
You will also be expected to have an appointment with your doctor at 6 weeks as is standard practice, and at 3 months if you find it necessary. The office visits will take approximately 20-30 minutes each.
Will there be additional charges or costs to me?
No, you and your insurance will only be billed for the procedure and the associated care.
There are no additional tests or office visits specifically for the research project. The only cost to you will be the time to fill out the questionnaires.
Do I have to participate in the research?
Although, we would appreciate if you participate, it is not a requirement that you participate in the research project to undergo the procedure using the TenJet device.
Will my privacy be protected?
Your personal information and privacy are protected per the HIPPA regulations. Patients are identified only using sequential numbers and letters on the forms and questionnaires associated with the research.
What do I need to do if I am interested?
Contact one of the participating physicians listed below to discuss your interest in the TenJet Patient Registry and if TenJet is the right treatment for you.
Participating Physicians
California
Eugene Roh, MD
Stanford Health Care
450 Broadway Street
Redwood City, CA
Phone: (650) 723-5256
https://stanfordhealthcare.org/doctors/r/yousik-roh.html
Florida
Michael Dakkak, DO
Cleveland Clinic Florida
2950 Cleveland Clinic Blvd
Weston, FL 33331
Phone: (877) 463-2010
https://my.clevelandclinic.org/staff/28993-michael-dakkak
Louisiana
Sean Bradley, MD
Ochsner Sports Medicine Institute, Ochsner Health
10310 The Grove Blvd
Baton Rouge, LA 70836
Phone: (225) 761-5200
https://www.ochsner.org/doctors/sean-bradley
Geoffrey Hogan, MD
Ochsner Sports Medicine
2400 South Burnside Avenue
Gonzales, LA 70737
Phone: (225) 761-5200
https://www.ochsner.org/services/sports-medicine-baton-rouge
North Carolina
David Berkoff, MD
UNC Orthopaedics
6011 Farrington Road
Chapel Hill, NC 27517
Phone: (919) 966-5760
https://www.uncortho.net/david-j-berkoff-md-nonoperative-sports-medicine/
Ohio
Jason Genin, DO
Cleveland Clinic Orthopaedic and Rheumatologic Institute
35000 Cleveland Clinic Blvd
Avon, OH 44011
Phone: (440) 260-3731
https://my.clevelandclinic.org/staff/15144-jason-genin
Vikas Patel, DO
Cleveland Clinic Orthopedic Institute
33300 Cleveland Clinic Blvd
Avon, OH 44011
Phone: (440) 695-4000
https://my.clevelandclinic.org/staff/22473-vikas-patel
Pennsylvania
Edward Rosero, DO
Rothman Orthopaedics
1200 Manor Drive
Chalfont, PA 18914
Phone: (800) 321-9999
https://rothmanortho.com/physicians/edward-rosero-do
Chris Varacallo, DO
Penn Highlands Healthcare Orthopedics and Sports Medicine
145 Hospital Avenue
DuBois, PA 15801
Phone: (814) 375-6200
https://www.phhealthcare.org/doctor/varacallo-christopher-p-do-caqsm-faafp-621
See more at ClinicalTrials.gov
IRB Approved at the Protocol Level
Nov 12, 2021